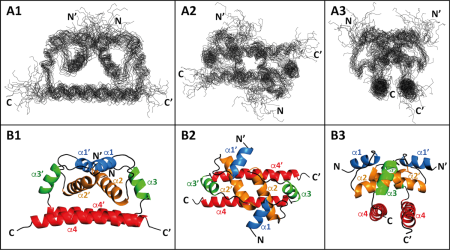
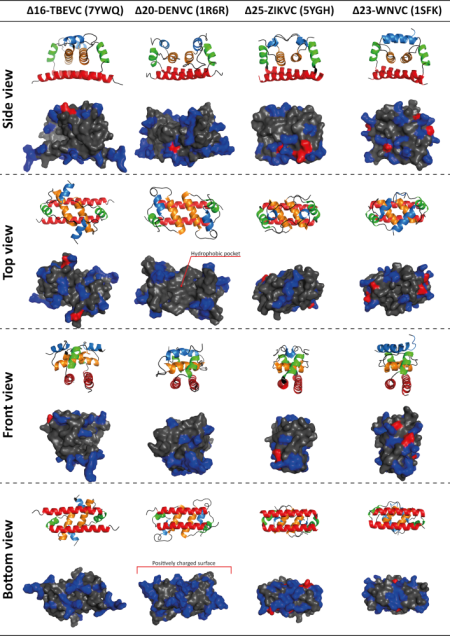
Flaviviruses such as Dengue virus (DENV), West Nile virus (WNV), Zika virus (ZIKV), Yellow fever virus (YFV), and Tick-borne encephalitis virus (TBEV) are important human pathogens belonging to the arthropod-borne viruses with a worldwide impact on human and animal health. Despite the availability of TBEV vaccine, there is still a need for tick-borne encephalitis treatment. Our research is focused on one of the promising targets for such antivirals, a flaviviral capsid (C) protein. The C protein triggers the flaviviral assembly, most probably by simultaneous binding the viral genomic RNA and ER lipid bilayer. Based on our recent publication reporting the NMR structure of TBEV C protein, we focused on the identification of the regions/residues of TBEV C involved in lipid-membrane binding and viral genomic RNA interactions. The obtained data should significantly expand our knowledge of the mechanism and specificity of C protein in the process of viral RNA packaging during TBEV infection and thus contribute to the development of targeted therapy.