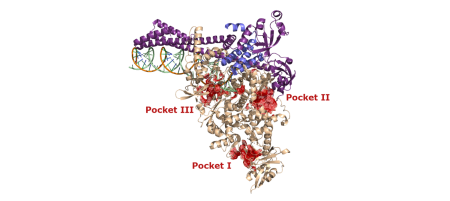
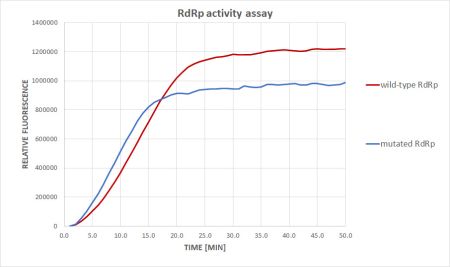
A novel coronavirus Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), emerged in late 2019 in Wuhan China. Since then, it has caused a global outbreak of acute respiratory disease associated with fatal pneumonia. Due to the intrinsic proofreading activity of the SARS-CoV-2 replication complex (RdRp) involved in the repair mechanisms of mutations arising during virus replication, the usage of nucleoside analogues as antiviral drugs for COVID-19 treatment is limited. Therefore, we focused on characterizing the putative allosteric sites of RdRp and screening the binding activity of small compounds, which would interfere with the allosteric modulation of RdRp. We pre-selected several hydrophobic pockets potentially involved in this process; and their characterization by a combination of mutagenesis and activity assay is currently in progress. Furthermore, we are interested in the in vitro analysis of naturally occurring mutations in the RdRp regions, whose occurrence relates to different SARS-CoV2 variants, which have been repeatedly reported in RdRp cofactors (nsp7, nsp8, and nsp12) over the last three years. Another target of our SARS-CoV-2 study is the exoribonuclease complex responsible for proofreading activity. The proofreading complex comprises the exoribonuclease domain (nsp14) and its cofactor nsp10. The intervention of proofreading during viral RNA replication is a promising approach to enhance the efficiency of active-site RdRp inhibitors.